Our proprietary technology enables the eye to generate its own therapeutic proteins.
Our novel non-viral gene therapy platform turns the eye into a production site for sustained delivery of therapeutic proteins by introducing proprietary DNA plasmids encoding therapeutic proteins directly into the ciliary muscle via our patented Electrotransfection System. Our unique approach offers the possibility of a wide range of targeted, long-lasting treatments that are safe, convenient, and effective.
All drug delivery approaches for retinal diseases have significant limitations:
- Intravitreal injections require frequent readministrations leading to fluctuating peak and trough drug levels.
- Viral vector gene therapy may require invasive subretinal surgery with a high risk of damage to the retina or other safety issues associated with viral vectors.
- Ocular implants involve introducing a foreign body into the eye and can result in corticosteroid-related ocular side effects.
- Systemic treatments have poor ocular bioavailability, require constant lab monitoring resulting in poor compliance, and can have significant side effects.
Eyevensys Electrotransfection System
The Electrotransfection System features 2 components, an electrical pulse generator and an ocular device, which deliver our proprietary plasmids into the ciliary muscle of the eye to set up the biofactories for intraocular therapeutic protein production.
The encoded plasmids are delivered into the ciliary muscle via a 3-step, minimally-invasive procedure.
Our Novel Non-viral Gene Therapy Approach Provides Treatment to the Entire Eye
By delivering the plasmids to the posterior ciliary muscle fibers, the expressed therapeutic proteins can easily reach all parts of the eye. They can reach the back of the eye by being secreted into the vitreous cavity and choroid—providing access to both sides of the retina—and into the front of the eye by being secreted into the aqueous humor.
Harnessing the Power of Electroporation
Our proprietary technology enables the eye to generate its own therapeutic proteins. Creating an electrical field around the injected area of the ciliary muscle using electroporation, the Electrotransfection System enables the plasmids to penetrate the smooth muscle cell membranes and reach the cell nuclei. The ciliary muscle cells then become biofactory sites, producing the encoded protein at therapeutic levels and providing sustained expression for 6 months or more.
Before
electric pulses application
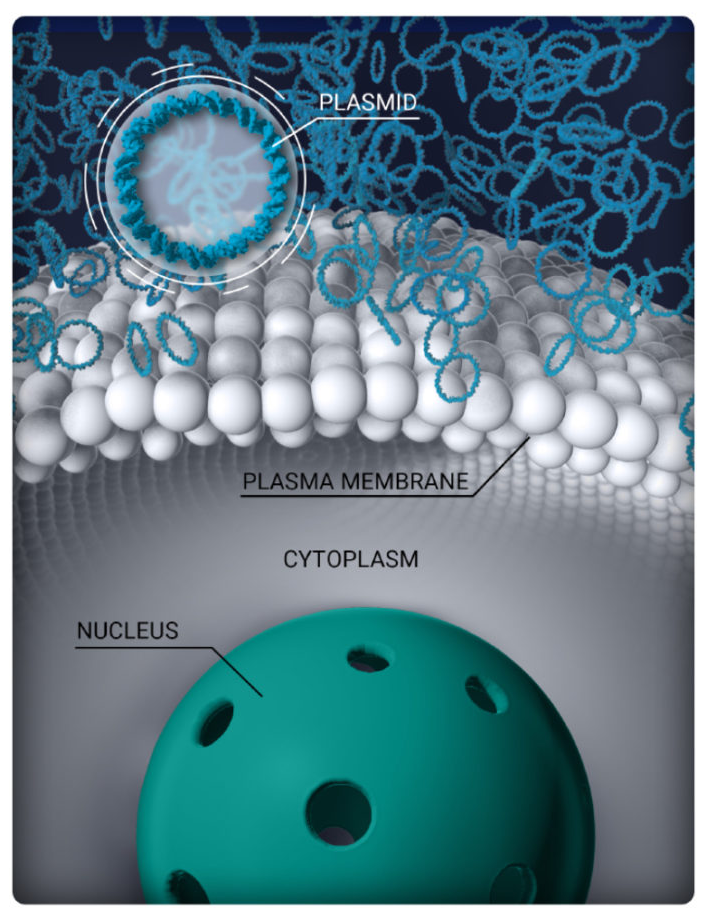
The cell membrane is not permeable.
Plasmids cannot enter the ciliary muscle cell.
During
electric pulses application

Electrical pulses make the ciliary muscle cell membrane permeable to the plasmids and push the plasmids into the cell towards the nucleus.
After
electric pulses application
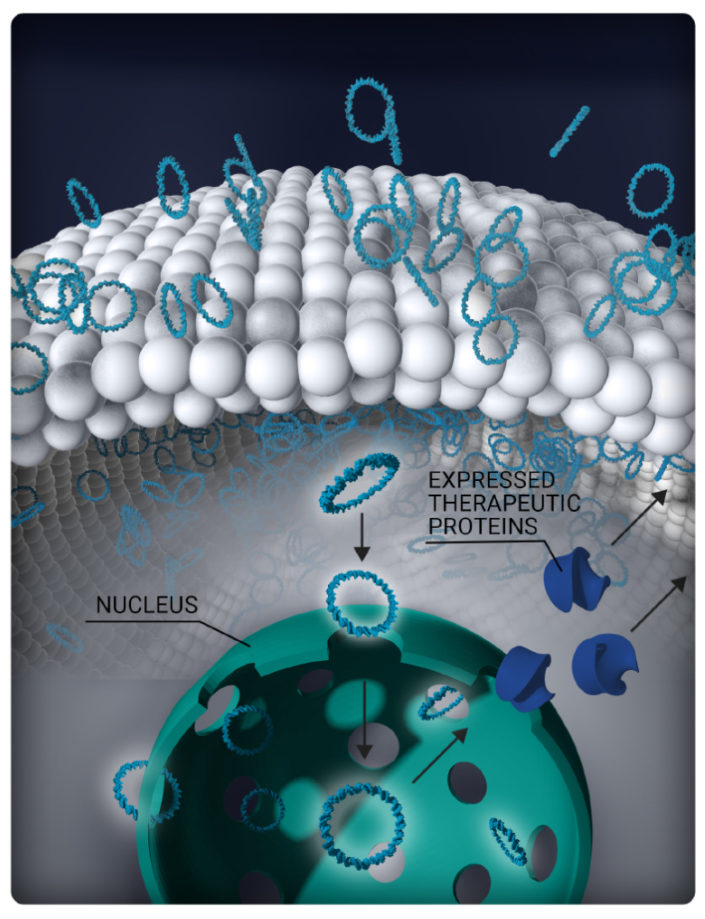
Plasmids enter the nucleus where the encoded gene is expressed and the produced therapeutic protein is then secreted into the vitreous and choroid to reach the back of the eye.
Advantages Over Existing Approaches
Safety
Avoids risks associated with more-invasive gene therapy options requiring subretinal injections, and reduces the risk of immunogenicity and carcinogenicity.
Convenience
Long-lasting therapeutic protein expression reduces the number of required treatments, making it more convenient for patients, their families, and ophthalmologists.
Wider Range of Treatment Options
Because DNA plasmids have no cargo size limitation, our technology expands the diversity of therapeutic proteins that can be delivered to the eye for the treatment of retinal diseases, by enabling the production of almost any secreted protein.Cost-effective
While manufacturing of viral vectors used in many traditional gene therapy approaches is a hurdle, our proprietary, cost-effective, and scalable manufacturing capacity allows for unlimited availability of our non-viral vectors to address the most common ocular indications.



