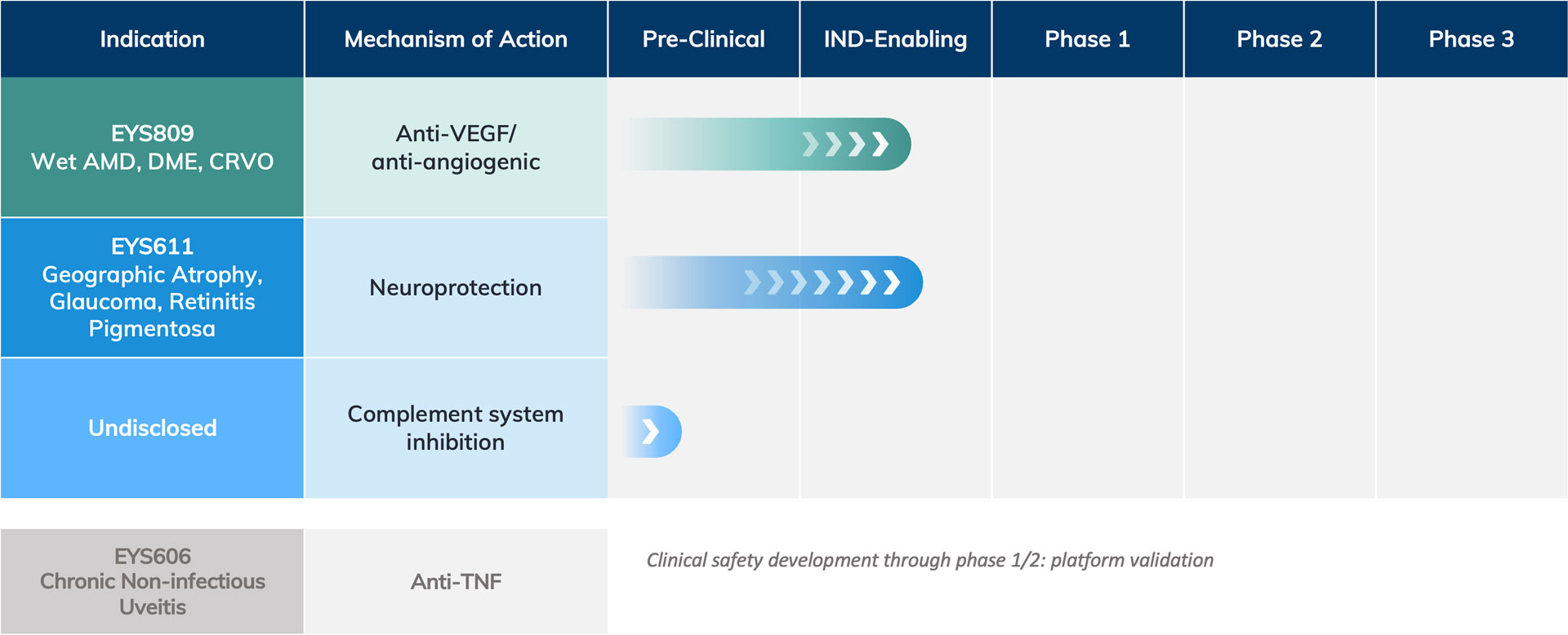
The Eyevensys pipeline, which leverages our proprietary platform technology, includes two key assets in development for two major indications: Wet Age-Related Macular Degeneration (AMD) and Geographic Atrophy, a late-stage form of Dry Age- Related Macular Degeneration. There is also upside for other future rare and prevalent indications in the retina including retinitis pigmentosa, diabetic macular edema, retinal vein occlusion, and glaucoma.
Completion of an earlier study of late-stage Non-Infectious Uveitis demonstrated the safety and feasibility of the Eyevensys platform.
Wide range of treatment options
Our unique non-viral gene therapy approach provides a wide range of treatment options with the potential to address a variety of ophthalmic diseases, both rare and common, some with no previously approved treatment. Unlike viral vectors, such as adeno-associated viruses, plasmids can carry almost unlimited cargo and can accommodate a variety of therapeutic proteins, including the largest ones, and without the safety issues observed with viral gene therapy.
The EYS809 program for the treatment of Wet AMD is the first priority for Eyevensys. With this therapy, a dual gene plasmid is encoded to express two therapeutic proteins— aflibercept, a well-known potent anti-VEGF, and decorin, a native protein, with antiangiogenic and antifibrotic characteristics. The EYS809 dual gene plasmid is injected in the ciliary muscle followed by Eyevensys’ electrotransfection procedure using an ocular device and a pulse generator specifically designed for the eye. The procedure lasts just a few seconds. Our expectation is to offer patients a treatment requiring less frequent re-treatment and better clinical outcomes.
The second priority for Eyevensys is the EYS611 program for treatment of geographic atrophy (the late stage of Dry AMD) using the same platform technology but with a plasmid encoded to express Transferrin, a native iron chelator with antioxidant and cytoprotective properties.
This program will also be developed for the treatment of retinitis pigmentosa, a rare form of retinal degeneration due to gene mutation.
Our expectation is to slow down the progression of these diseases and prevent associated vision loss.
The technology platform has been evaluated for safety and feasibility in patients with late-stage chronic non-infectious uveitis (CNIU) using a plasmid encoded to express a TNF-alpha trap protein (EYS606 project).
This validation program has also allowed Eyevensys to optimize the design of the ocular device with the support of retina experts.
Consequently, Eyevensys has secured manufacturing partnerships with two reknowned experts in the fields of ophthalmic medical devices and electrotransfection: Philips-Medisize, a Molex company, and Minnetronix.
EYS606 is currently available for licensing.
Targeting extracellular retinal disease pathways
Eyevensys’ current pipeline includes plasmids encoding therapeutic proteins that target extracellular retinal disease pathways, such as proteins targeting the complement system for the treatment of dry AMD, and proteins targeting extracellular matrix (ECM) to treat glaucoma. The Eyevensys technology is also amenable to antibody gene therapy programs to produce in-situ consistent levels of therapeutic antibodies to address, among others, retinal vascular diseases, and macular edemas (e.g., diabetic retinopathy, diabetic macular edema, and retinal vein occlusion).
