Validating Studies with EYS606
EYS606 is a novel non-viral gene therapy approach that was developed for the treatment of chronic non-infectious uveitis (CNIU). CNIU, which meets the criteria for a rare disease (affecting 250,000 patients in North America and Europe), is a sight-threatening immune-mediated inflammatory disease that causes inflammation in the eye that can lead to severely reduced vision or blindness resulting from ocular tissue damage. Uveitis affects people of all ages and may be triggered by another underlying condition.
The therapy has been used to validate the Eyevensys Electrotransfection System for safety and feasibility in two clinical trial studies across Europe and the U.S. with ongoing follow-up. As a result of data gathered in both studies, Eyevensys’ proprietary technology has been shown safe and the drug delivery method has been proven to be successful. The final design of the device will leverage data gathered during these studies.
People with chronic non-infectious uveitis in the US
Uveitis can be classified as anterior, intermediate, posterior, or panuveitis, on the basis of the part of the eye that is affected. Patients with CNIU commonly have uveitis involving the back of the eye (intermediate, posterior, and panuveitis), placing them at higher risk for vision loss due to disease exacerbations and complications of intraocular inflammation.
Current treatment options for CNIU include corticosteroids (systemic or injections in or around the eye), systemic immunosuppressants, or biologics such as systemic tumor necrosis factor (TNF) inhibitors. These standard-of-care treatments can have considerable side effects, particularly when used long term. Hence, there is a strong need for more convenient, safer, and effective treatment options for patients with CNIU.
EYS606 combines plasmids encoding a potent fusion protein that neutralizes the activity of TNF-α and the proprietary Eyevensys Electrotransfection System used to introduce the plasmids into the eye. TNF is a cytokine that has been shown to play a pivotal role in mediating intraocular inflammation in CNIU.
EYS606 has already received orphan designation in Europe and is being investigated in 2 ongoing clinical trials enrolling patients with CNIU. Early results from these trials reveal no serious safety concerns and suggest that the treatment effect may persist for more than 6 months after a single administration.
EYS606 is currently available for licensing.
ELECTRO STUDY
EYS606-CT2 Trial (US)
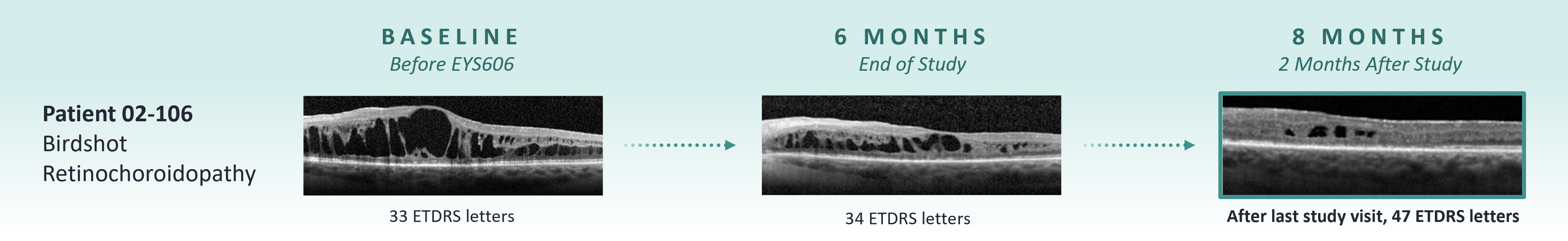
The Electro Study was a phase 2, open-label, multicenter, randomized trial designed to assess the efficacy and safety of EYS606 in patients with chronic non-infectious uveitis. Three participants received two administrations of EYS606 and two completed the study at week 48. Throughout the study, participants experienced mild to moderate ocular adverse events, including conjunctival hemorrhage, visual impairment, lenticular opacity, cataract, retinal vasculitis, and macular edema. None of the reported adverse events were severe, serious, or related to the study drug, and all adverse events were resolved by the end of the study. Two participants showed a clinical response, and one maintained the treatment effect until the end of the study.

EYS606-CT1 Trial (EU)
A phase 1/2, open-label, multicenter, dose-escalation study was conducted to assess the safety and tolerability of EYS606 in patients with chronic non-infectious uveitis. The study found that treatment with EYS606 led to primarily mild ocular treatment-emergent adverse events (TEAEs) with no instances of serious adverse events. The most frequently observed ocular TEAEs included conjunctival hemorrhage (60%) and transient reductions in visual acuity (46.7%). Among the seven participants eligible for clinical activity assessment, four (57.1%) exhibited a positive response to treatment. Notably, two of the responders maintained clinical response at week 24. Additionally, one responder who received a second treatment due to a loss of clinical effect at week 24 experienced recurrent vision improvement that persisted through the study’s conclusion at week 48. No participants experienced a worsening of vision following EYS606 treatment.
Learn more about our phase 1/2 open-label dose-escalation study.
